Promoting Safe and Appropriate Medicine Use in Sierra Leone
Promoting Safe and Appropriate Medicine Use in Sierra Leone
by JoAnn Paradis and Stacy Lu

Irrational medicine use and poor pharmaceutical management are widespread problems throughout all levels of Sierra Leone’s health system. Misuse, underuse, and overuse of medicines are particularly worrying because they contribute to the rise of antimicrobial resistance (AMR) and threaten the effective prevention and treatment of infections caused by bacteria, parasites, and viruses.
Recognizing that coordinated action is needed to minimize the emergence and spread of AMR, Management Sciences for Health (MSH) has catalyzed multidisciplinary and cross-sectoral coalitions to build awareness of the threat of AMR and advocate for its containment.
As part of its post-Ebola recovery work to strengthen its pharmaceutical system, Sierra Leone’s Directorate of Drugs and Medical Supplies (DDMS) partnered with the US Agency for International Development-funded Systems for Improved Access to Pharmaceutical and Services (SIAPS) Program, implemented by MSH, to develop efficient procurement, distribution, and inventory systems and establish stakeholder coordination and oversight mechanisms known as hospital Drug and Therapeutics Committees (DTCs).
DTCs are a proven way to strengthen health systems and reduce practices that lead to AMR by stemming inappropriate medicine use and promoting sound management among health care professionals. A DTC provides a forum for evidence-based practice reviews and transparent oversight of pharmaceutical management. It manages medicine selection and procurement, promotes good prescribing and dispensing practices, and implements strategies to improve medicine use throughout a health care facility. DTCs also make decisions on a health facility’s reported adverse drug reactions and are a part of the capacity building cycle designed to inform national priority setting, target resources, and track progress.
Within five months, from November 2016 to March 2017, SIAPS and the DDMS conducted a rapid baseline assessment on tracer medicines in four hospitals; used the findings of the assessment to conduct a DTC familiarization and establishment workshop; drafted terms of reference for establishing and operationalizing DTCs; and launched groups across four hospitals: Connaught Tertiary, Ola During Children’s, Makeni Government, and Princes Christian Maternity. Six additional hospitals across the country are on track to establish DTCs before the end of 2017.
The DTCs in Sierra Leone have achieved a number of important milestones toward AMR containment through improved pharmaceutical management.
Supplying data through an electronic treatment register
A significant factor of success in Sierra Leone’s DTC operations was the development of and orientation to an electronic treatment register (eTR). The eTR was developed to capture critical information on patients and their prescription and use of medicines, enabling health facilities to better manage and make decisions about their supply. The eTR is easy to use and can efficiently aggregate, summarize, and print relevant data to inform decision making.
Conducting regular rapid reviews to measure rational medicine use
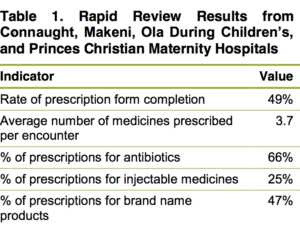
In November 2016 and again in August 2017, SIAPS supported four DTCs in conducting a rapid review of prescriptions to inform the status against select indicators on rational medicine use. Regular DTC reviews help identify areas for improvement that the committees can address through prescriber, dispenser, and patient partnerships. The first two reviews measured antibiotic prescribing, injectable medicine use, brand versus generic medicines, number of medicines prescribed per encounter, and average rate of prescription forms that include all required information.
Enhancing data gathering and security through a new prescription format
The lack of standardized, preprinted prescription templates is a major challenge to many health facilities in Sierra Leone, hampering efficiency and effective data gathering. Prescriptions are critical, official documents of the health system and require secure storage. They provide data that can be analyzed to yield key patient and product information, as well as prescribing and dispensing data for rational medicine use initiatives. In consultation with the DDMS, the DTCs have taken the initiative to revise and use the standardized prescription template. Six hospitals have introduced new prescription forms to guide the collection of information around medicine use.
Improving access to affordable, quality products through an in-hospital pharmacy
![[Pharmacist Tamba M.D. Saquee explains how Makeni Government Hospital’s DTC supported the establishment of a cost recovery pharmacy at the hospital.] {Photo Credit: Gabriel Daniel/MSH}](https://msh.org/wp-content/uploads/2017/11/gabriel_daniel_photo_2.jpg)
Hospital DTCs can facilitate planning for other areas of hospital improvement. For example, Makeni Government Hospital financed and established a cost-recovery unit within its pharmacy department, supporting patients who did not have access to medicines at the hospital’s pharmacy through the government’s health care initiative, which offers free medicines and related supplies to pregnant women and children under the age of five. The establishment of a cost-recovery pharmacy within the hospital helped ensure more equitable access to safe, effective, and accurately dispensed medicines, completing the cycle of quality services, treatment, and case management. The income generated from the sale of medicines can also help the hospital further improve patient services.
Ongoing commitment to fight AMR
The government of Sierra Leone, committed to combatting the threat of AMR, is developing a national action plan to ensure the appropriate use of medicines and essential supplies. The newly established DTCs continue to advocate for progress on this action plan and are supporting the DDMS and Pharmacy Board of Sierra Leone to develop hospital formularies.